Silica sand Chemistry
Silicon dioxide, also known as silica (from the Latin silex), is a chemical compound that is an oxide of silicon with the chemical formula SiO2. It has been known since ancient times. Silica is most commonly found in nature as quartz, as well as in various living organisms.
In many parts of the world, silica is the major constituent of sand it is one of the most complex and most abundant families of materials, existing both as several minerals and being produced synthetically.
Notable examples include fused quartz, crystal, fumed silica, silica gel, and aerogels.
Applications range from structural materials to microelectronics to components used in the food industry.
Production

Silica sand quarry
Silicon dioxide is mostly obtained by mining and purification of quartz. Quartz comprises more than 10% by mass of the earth’s crust.
This product will be suitable for many purposes while for others chemical processing will be required to make a purer or otherwise more suitable (e.g. more reactive or fine-grained) product.



Images of washed silica sand after mining
Other forms of Silica Production
- Fumed silica
Pyrogenic silica (sometimes called fumed silica or silica fume) is a very fine particulate or colloidal form of silicon dioxide. It is prepared by burning SiCl4 in an oxygen rich hydrocarbon flame to produce a “smoke” of SiO2 below is the reaction chemical equation
SiCl4 + 2 H2 + O2 → SiO2 + 4 HCl.
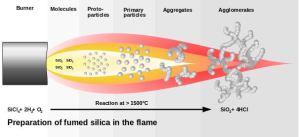
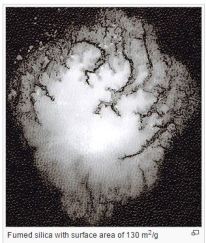
Image: Preparation of fumed silica flame. Image of Fumed Silica
Silica fume

This product is obtained as byproduct from hot processes like ferro-silicon production. It is less pure than fumed silica and should not be confused with that product. The production process, particle characteristics and fields of application of fumed silica are all different from those of silica fume.
Precipitated silica
Amorphous silica, silica gel, is produced by the acidification of solutions of sodium silicate. The gelatinous precipitate is first washed and then dehydrated to produce colorless microporous silica. Idealized equation involving a trisilicate and sulfuric acid is shown:
Na2Si3O7 + H2SO4 → 3 SiO2 + Na2SO4 + H2O
Approximately one billion kilograms/year (1999) of silica was produced in this manner, mainly for use for polymer composites – tires and shoe soles.
On microchips
Thin films of silica grow spontaneously on silicon wafers via thermal oxidation. This route gives a very shallow layer (approximately 1nm or 10 Å of so-called native oxide.Higher temperatures and alternative environments are used to grow well-controlled layers of silicon dioxide on silicon, for example at temperatures between 600 and 1200 °C, using so-called dry or wet oxidation with O2 or H2O, respectively. The depth of the layer of silicon replaced by the dioxide is 44% of the depth of the silicon dioxide layer produced.
The native oxide layer can be beneficial in microelectronics, where it acts as electric insulators with high chemical stability. In electrical applications, it can protect the silicon, store charge, block current, and even act as a controlled pathway to limit current flow.
Laboratory or specialty methods
From silicate esters
Many routes to silicon dioxide start with silicate esters, the best known being tetraethyl orthosilicate (TEOS). Simply heating TEOS at 680–730 °C gives the dioxide:
Si(OC2H5)4 → SiO2 + 2 O(C2H5)2
Similarly TEOS combusts around 400 °C:
Si(OC2H5)4 + 12 O2 → SiO2 + 10 H2O + 8 CO2
TEOS undergoes hydrolysis via the so-called sol-gel process. The course of the reaction and nature of the product are affected by catalysts, but the idealized equation is
Si(OC2H5)4 + 2 H2O → SiO2 + 4 HOCH2CH3
Other methods]
Being highly stable, silicon dioxide arises from many methods. Conceptually simple, but of little practical value, combustion of silane gives silicon dioxide. This reaction is analogous to the combustion of methane:
SiH4 + 2 O2 → SiO2 + 2 H2O.
Uses
An estimated 95% of silicon dioxide produced is consumed in the construction industry, e.g. for the production of Portland cement. Other major applications are listed below.
Precursor to glass and silicon
Silica is used primarily in the production of glass for windows, drinking glasses, beverage bottles, and many other uses. The majority of optical fibers for telecommunication are also made from silica. It is a primary raw material for many ceramics such as earthenware, stoneware, and porcelain.
Silicon dioxide is used to produce elemental silicon. The process involves carbothermic reduction in an electric arc furnace:
SiO2 + 2 C → Si + 2 CO
Major component used in sand casting
Silica, in the form of sand is used as the main ingredient in sand casting for the manufacture of a large number of metallic components in engineering and other applications. The high melting point of silica enables it to be used in such applications.
Food and pharmaceutical applications
Silica is a common additive in the production of foods, where it is used primarily as a flow agent in powdered foods, or to adsorb water in hygroscopic applications. It is the primary component of diatomaceous earth. Colloidal silica is also used as a wine, beer, and juice fining agent.
In pharmaceutical products, silica aids powder flow when tablets are formed.
Other
A silica-based aerogel was used in the Stardust spacecraft to collect extraterrestrial particles. Silica is also used in the extraction of DNA and RNA due to its ability to bind to the nucleic acids under the presence of chaotropes. Hydrophobic silica is used as a defoamer component. In hydrated form, it is used in toothpaste as a hard abrasive to remove tooth plaque.
In its capacity as a refractory, it is useful in fiber form as a high-temperature thermal protection fabric. In cosmetics, it is useful for its light-diffusing properties and natural absorbency. It is also used as a thermal enhancement compound in ground source heat pump industry.
Structure

Structural motif found in α-quartz, but also found in almost all forms of silicon dioxide.
In the majority of silicates, the Si atom shows tetrahedral coordination, with 4 oxygen atoms surrounding a central Si atom. The most common example is seen in the quartz crystalline form of silica SiO2. In each of the most thermodynamically stable crystalline forms of silica, on average, all 4 of the vertices (or oxygen atoms) of the SiO4 tetrahedra are shared with others, yielding the net chemical formula: SiO2.
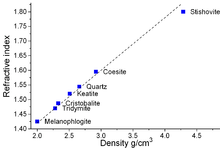
Relation between refractive index and density for some SiO2 forms.
For example, in the unit cell of α-quartz, the central tetrahedron shares all 4 of its corner O atoms, the 2 face-centered tetrahedra share 2 of their corner O atoms, and the 4 edge-centered tetrahedra share just one of their O atoms with other SiO4 tetrahedra. This leaves a net average of 12 out of 24 total vertices for that portion of the 7 SiO4 tetrahedra that are considered to be a part of the unit cell for silica
SiO2 has a number of distinct crystalline forms (polymorphs) in addition to amorphous forms. With the exception of stishovite and fibrous silica, all of the crystalline forms involve tetrahedral SiO4 units linked together by shared vertices in different arrangements. Silicon–oxygen bond lengths vary between the different crystal forms, for example in α-quartz the bond length is 161 pm, whereas in α-tridymite it is in the range 154–171 pm. The Si-O-Si angle also varies between a low values of 140° in α-tridymite, up to 180° in β-tridymite. In α-quartz the Si-O-Si angle is 144°.
Fibrous silica has a structure similar to that of SiS2 with chains of edge-sharing SiO4 tetrahedra. Stishovite, the higher-pressure form, in contrast has a rutile-like structure where silicon is 6-coordinate. The density of stishovite is 4.287 g/cm3, which compares to α-quartz, the densest of the low-pressure forms, which has a density of 2.648 g/cm3. The difference in density can be ascribed to the increase in coordination as the six shortest Si-O bond lengths in stishovite (four Si-O bond lengths of 176 pm and two others of 181 pm) are greater than the Si-O bond length (161 pm) in α-quartz.] The change in the coordination increases the ionicity of the Si-O bond.[17] But more important is the observation that any deviations from these standard parameters constitute microstructural differences or variations, which represent an approach to an amorphous, vitreous or glassy solid.
The only stable form under normal conditions is α-quartz and this is the form in which crystalline silicon dioxide is usually encountered. In nature impurities in crystalline α-quartz can give rise to colors (see list). The high temperature minerals, cristobalite and tridymite, have both a lower density and index of refraction than quartz. Since the composition is identical, the reason for the discrepancies must be in the increased spacing in the high temperature minerals. As is common with many substances, the higher the temperature the farther apart the atoms due to the increased vibration energy.
The transformation from α-quartz to beta-quartz takes place abruptly at 573 C. Since the transformation is accompanied by a significant change in volume it can easily induce fracturing of ceramics or rocks passing through this temperature limit.
The high-pressure minerals, seifertite, stishovite, and coesite, on the other hand, have a higher density and index of refraction when compared to quartz. This is probably due to the intense compression of the atoms that must occur during their formation, resulting in a more condensed structure.
Faujasite silica is another form of crystalline silica. It is obtained by dealumination of a low-sodium, ultra-stable Y zeolite with a combined acid and thermal treatment. The resulting product contains over 99% silica, has high crystallinity and high surface area (over 800 m2/g). Faujasite-silica has very high thermal and acid stability. For example, it maintains a high degree of long-range molecular order (or crystallinity) even after boiling in concentrated hydrochloric acid.
Molten silica exhibits several peculiar physical characteristics that are similar to the ones observed in liquid water: negative temperature expansion, density maximum (at temperatures ~5000 °C), and a heat capacity minimum. Its density decreases from 2.08 g/cm3 at 1950 °C to 2.03 g/cm3 at 2200 °C. When molecular silicon monoxide, SiO, is condensed in an argon matrix cooled with helium along with oxygen atoms generated by microwave discharge, molecular SiO2 is produced with a linear structure. Dimeric silicon dioxide, (SiO2)2 has been prepared by reacting O2 with matrix isolated dimeric silicon monoxide, (Si2O2). In dimeric silicon dioxide there are two oxygen atoms bridging between the silicon atoms with an Si-O-Si angle of 94° and bond length of 164.6 pm and the terminal Si-O bond length is 150.2 pm. The Si-O bond length is 148.3 pm, which compares with the length of 161 pm in α-quartz. The bond energy is estimated at 621.7 kJ/mol.
Fused quartz
When molten silicon dioxide SiO2 is rapidly cooled, it does not crystallize but solidifies as a glass. The geometry of the silicon and oxygen centers in glass is similar to that in quartz and most other crystalline forms of the same composition, i.e., silicon is surrounded by a regular tetrahedra of oxygen centers. The difference between the glass and the crystalline forms arise from the connectivity of these tetrahedral units. Although there is no long range periodicity in the glassy network there remains significant ordering at length scales well beyond the SiO bond length. One example of this ordering is found in the preference of the network to form rings of 6-tetrahedra.
The glass transition temperature of pure SiO2 is about 1475 K. = 1201.850c
Chemical reactions

Manufactured silica fume at maximum surface area of 380 m2/g
Silica is converted to silicon by reduction with carbon.
Fluorine reacts with silicon dioxide to form SiF4 and O2 whereas the other halogen gases (Cl2, Br2, I2) are essentially unreactive.
Silicon dioxide is attacked by hydrofluoric acid (HF) to produce hexafluorosilicic acid:
SiO2 + 6 HF → H2SiF6 + 2 H2O.
HF is used to remove or pattern silicon dioxide in the semiconductor industry.
Silicon dioxide dissolves in hot concentrated alkali or fused hydroxide, as described in this idealized equation:
SiO2 + 2 NaOH → Na2SiO3 + H2O.
Silicon dioxide reacts with basic metal oxides (e.g. sodium oxide, potassium oxide, lead(II) oxide, zinc oxide, or mixtures of oxides forming silicates and glasses as the Si-O-Si bonds in silica are broken successively). As an example the reaction of sodium oxide and SiO2 can produce sodium orthosilicate, sodium silicate, and glasses, dependent on the proportions of reactants:
2 Na2O + SiO2 → Na4SiO4;
Na2O + SiO2 → Na2SiO3;
(0.25–0.8)Na2O + SiO2 → glass.
Examples of such glasses have commercial significance, e.g. soda-lime glass, borosilicate glass, lead glass. In these glasses, silica is termed the network former or lattice former.

Bundle of optical fibers composed of high purity silica.
Silicon dioxide reacts in heated reflux under nitrogen with ethylene glycol and an alkali metal base to produce highly reactive,pentacoordinate silicates which provide access to a wide variety of new silicon compounds. The silicates are essentially insoluble in all polar solvent except methanol.
Silicon dioxide reacts with elemental silicon at high temperatures to produce SiO:
SiO2 + Si → 2 SiO
Solubility in water
The solubility of silicon dioxide in water strongly depends on its crystalline form and is 3–4 times higher for silica than quartz; as a function of temperature, it peaks at about 340 °C.This property is used to grow single crystals of quartz in a hydrothermal process where natural quartz is dissolved in superheated water in a pressure vessel that is cooler at the top. Crystals of 0.5–1 kg can be grown over a period of 1–2 months. These crystals are a source of very pure quartz for use in electronic applications.
Occurrence
Biology
Even though it is poorly soluble, silica occurs widely in many plants. Plant materials with high silica phytolith content appear to be of importance to grazing animals, from chewing insects to ungulates. Studies have shown that it accelerates tooth wear, and high levels of silica in plants frequently eaten by herbivores may have developed as a defense mechanism against predation.
It is also the primary component of rice husk ash, which is used, for example, in filtration and cement manufacturing.
Silicification in and by cells has been common in the biological world for well over a billion years. In the modern world it occurs in bacteria, single-celled organisms, plants, and animals (invertebrates and vertebrates). Prominent examples include:
- Tests or frustules (i.e. shells) of diatoms, Radiolaria and testate amoebae.
- Silica phytoliths in the cells of many plants, including Equisetaceae, practically all grasses, and a wide range of dicotyledons.
- The spicules forming the skeleton of many sponges.
Crystalline minerals formed in the physiological environment often show exceptional physical properties (e.g., strength, hardness, fracture toughness) and tend to form hierarchical structures that exhibit microstructural order over a range of scales. The minerals are crystallized from an environment that is undersaturated with respect to silicon, and under conditions of neutral pH and low temperature (0–40 °C).
Formation of the mineral may occur either within the cell wall of an organism (such as with phytoliths), or outside the cell wall, as typically happens with tests. Specific biochemical reactions exist for mineral deposition. Such reactions include those that involve lipids, proteins, and carbohydrates.
It is unclear in what ways silica is important in the nutrition of animals. This field of research is challenging because silica is ubiquitous and in most circumstances dissolves in trace quantities only. All the same it certainly does occur in the living body, leaving us with the problem that it is hard to create proper silica-free controls for purposes of research. This makes it difficult to be sure when the silica present has had operative beneficial effects, and when its presence is coincidental, or even harmful. The current consensus is that it certainly seems important in the growth, strength, and management of many connective tissues. This is true not only for hard connective tissues such as bone and tooth but possibly in the biochemistry of the subcellular enzyme-containing structures as well.
Health effects

Quartz sand (silica) as main raw material for commercial glass production
Silica ingested orally is essentially nontoxic, with an LD50 of 5000 mg/kg (5 g/kg). On the other hand, inhaling finely divided crystalline silica dust can lead to silicosis, bronchitis, or cancer, as the dust becomes lodged in the lungs and continuously irritates them, reducing lung capacities. Studies of workers with exposure to crystalline silica have shown 10-fold higher than expected rates of lupus and othersystemic autoimmune diseases compared to expected rates in the general population. Prior to new rules issued in 2013, OSHA allowed 100 µg per cubic meter of air. The new regulations reduce the amount to 50 µg/m3 down from 100 µg/m3. The exposure limit for the construction industry is also set at 50 µg/m3 down from 250 µg/m3.
In the body crystalline silica particles do not dissolve over clinically relevant periods. Silica crystals inside the lungs can activate the NLRP3 inflammasome inside macrophages and dendritic cells and thereby result in processing of pro-Interleukin 1 beta into its mature form. Chronic exposure to silica may thereby account for some of its health hazards, as interleukin-1 is a highly pro-inflammatory cytokine in the immune system. This effect can create an occupational hazard for people working with sandblasting equipment, products that contain powdered crystalline silica and so on. Children, asthmatics of any age, allergy sufferers, and the elderly (all of whom have reduced lung capacity) can be affected in much less time. Amorphous silica, such as fumed silica is not associated with development of silicosis, but may cause irreversible lung damage in some cases.
Laws restricting silica exposure with respect to the silicosis hazard specify that they are concerned only with silica that is both crystalline and dust-forming.
A study that followed subjects for 15 years found that higher levels of silica in water appeared to decrease the risk of dementia. The study found an association between an increase of 10 milligram-per-day of the intake of silica in drinking water with a decreased risk of dementia of 11%.
Crystalline silica is used in hydraulic fracturing of formation which contain tight oil and shale gas, a use which presents a health hazard to workers. In 2013 OSHA announced tightened restrictions on the amount of crystalline silica which could be present and required “green completion” of fracked wells to reduce exposure.[31] Crystalline silica is an occupational hazard for those working with stone countertops, because the process of cutting and installing the countertops creates large amounts of airborne silica.[37]
Crystalline forms
SiO2, more so than almost any material, exists in many crystalline forms (called polymorphs).
| CRYSTALLINE FORMS OF SIO2 | |||||
| Form | Crystal symmetry Pearson symbol, group No. | Density g/cm3 | Notes | Structure | |
| Form | Crystal symmetry Pearson symbol, group No. | ρ g/cm3 | Notes | Structure |
| α-quartz | rhombohedral (trigonal) hP9, P3121 No.152 | 2.648 | Helical chains making individual single crystals optically active; α-quartz converts to β-quartz at 846 K | 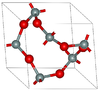 |
| β-quartz | hexagonal hP18, P6222, No. 180 | 2.533 | Closely related to α-quartz (with an Si-O-Si angle of 155°) and optically active; β-quartz converts to β-tridymite at 1140 K | 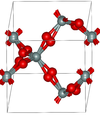 |
| α-tridymite | orthorhombic oS24, C2221, No.20[ | 2.265 | Metastable form under normal pressure | 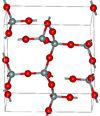 |
| β-tridymite | hexagonal hP12, P63/mmc, No. 194 | Closely related to α-tridymite; β-tridymite converts to β-cristobalite at 2010 K | 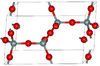 | |
| α-cristobalite | tetragonal tP12, P41212, No. 92 | 2.334 | Metastable form under normal pressure | 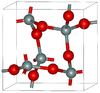 |
| β-cristobalite | cubic cF104, Fd3m, No.227 | Closely related to α-cristobalite; melts at 1978 K |  | |
| keatite | tetragonal tP36, P41212, No. 92 | 3.011 | Si5O10, Si4O14, Si8O16 rings; synthesised from glassy silica and alkali at 600–900 K and 40–400 MPa |  |
| moganite | monoclinic mS46, C2/c, No.15 | Si4O8 and Si6O12 rings |  | |
| coesite | monoclinic mS48, C2/c, No.15 | 2.911 | Si4O8 and Si8O16 rings; 900 K and 3–3.5 GPa |  |
| stishovite | Tetragonal tP6, P42/mnm, No.136 | 4.287 | One of the densest (together with seifertite) polymorphs of silica; rutile-like with 6-fold coordinated Si; 7.5–8.5 GPa | 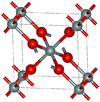 |
| seifertite | orthorhombic oP, Pbcn[47] | 4.294 | One of the densest (together with stishovite) polymorphs of silica; is produced at pressures above 40 GPa. | 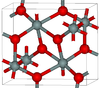 |
| melanophlogite | cubic (cP*, P4232, No.208) or tetragonal (P42/nbc) | 2.04 | Si5O10, Si6O12 rings; mineral always found with hydrocarbons in interstitial spaces – a clathrasil |  |
| faujasite | cubic cF576, Fd3m, No.227 | 1.92 | Sodalite cages connected by hexagonal prisms; 12-membered ring pore opening; faujasite structure. | 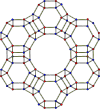 |
| fibrous W-silica | orthorhombic oI12, Ibam, No.72 | 1.97 | Like SiS2 consisting of edge sharing chains, melts at ~1700 K | 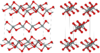 |
| 2D silica | hexagonal | Sheet-like bilayer structure | 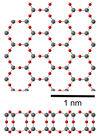 | |









No comments