Azeotrope Chemistry
An azeotrope
or a constant boiling mixture is a mixture of two or more liquids whose
proportions cannot be altered by simple distillation. This happens because
when an
azeotrope is boiled, the vapour has the same proportions of constituents as the unboiled mixture.
azeotrope is boiled, the vapour has the same proportions of constituents as the unboiled mixture.
Because
their composition is unchanged by distillation, azeotropes are also called
(especially in older texts) constant boiling mixtures. The word azeotrope is
derived from the Greek words ζέειν (boil) and τρόπος (turning) combined with
the prefix α- (no) to give the overall meaning, "no change on boiling".
The term "azeotrope" was coined in 1911 by English chemist John Wade
(1864–1912) and Richard William Merriman.
Many
azeotropic mixtures of pairs of compounds are known, and many azeotropes of
three or more compounds are also known. In such a case it is not possible to
separate the components by fractional distillation. There are two types of
azeotropes: minimum boiling azeotrope and maximum boiling azeotrope. A solution
that shows greater positive deviation from Raoult's law forms a minimum boiling
azeotrope at a specific composition. For example, an ethanol-water mixture
(obtained by fermentation of sugars) on fractional distillation yields a
solution containing approximately 95% by volume of ethanol. Once this
composition has been achieved, the liquid and vapour have the same composition,
and no further separation occurs. A solution that shows large negative
deviation from Raoult's law forms a maximum boiling azeotrope at a specific
composition. Nitric acid and water is an example of this class of azeotrope.
This azeotrope has an approximate composition of 68% nitric acid and 32% water
by mass, with a boiling point of 393.5 K.
Positive and negative azeotropes
Each
azeotrope has a characteristic boiling point. The boiling point of an
azeotrope is either less than the boiling point temperatures of any of its
constituents (a positive azeotrope), or greater than the boiling point of any
of its constituents (a negative azeotrope).
A well-known
example of a positive azeotrope is 95.63% ethanol and 4.37% water (by weight). Ethanol boils at 78.4 °C,
water boils at 100 °C, but the azeotrope boils at 78.2 °C, which is
lower than either of its constituents.
Indeed,
78.2 °C is the minimum temperature at which any ethanol/water solution can
boil at atmospheric pressure. In general, a positive azeotrope boils at a lower
temperature than any other ratio of its constituents. Positive azeotropes are
also called minimum boiling mixtures or pressure
maximum azeotropes.
An example
of a negative azeotrope is hydrochloric acid at a concentration of
20.2% and 79.8% water (by weight).
Hydrogen chloride boils at −84 °C and
water at 100 °C, but the azeotrope boils at 110 °C, which is higher
than either of its constituents.
The maximum temperature at which any
hydrochloric acid solution can boil is 110 °C. In general, a negative
azeotrope boils at a higher temperature than any other ratio of its
constituents.
Negative azeotropes are also called maximum boiling
mixtures or pressure minimum azeotropes.
Homogeneous
and heterogeneous azeotropes
If the
constituents of a mixture are not completely miscible, an azeotrope can be
found inside the miscibility gap. This type of azeotrope is called
heterogeneous azeotrope. If the azeotropic composition is outside the
miscibility gap or the constituents of the mixture are completely miscible, the
type of azeotrope is called a homogeneous azeotrope. A heteroazeotropic
distillation (see heteroazeotrope) will have two liquid phases.
Number of
constituents
Azeotropes
consisting of two constituents, such as the two examples above, are
called binary azeotropes. Those consisting of three
constituents are called ternary azeotropes. Azeotropes of more
than three constituents are also known.
Zeotropy
Combinations
of solvents that do not form an azeotrope when mixed in any proportion are said
to be zeotropic.
Distillation of mixtures
If two
solvents can form a positive azeotrope, then distillation of any mixture of
those constituents will result in the distillate being closer in
composition to the azeotrope than the starting mixture. For example, if a 50/50
mixture of ethanol and water is distilled once, the distillate will be 80%
ethanol and 20% water, which is closer to the azeotropic mixture than the
original. Distilling the 80/20% mixture produces a distillate that is 87%
ethanol and 13% water. Further repeated distillations will produce mixtures
that are progressively closer to the azeotropic ratio of 95.5/4.5%. No numbers
of distillations will ever result in a distillate that exceeds the azeotropic
ratio. Likewise, when distilling a mixture of ethanol and water that is richer
in ethanol than the azeotrope, the distillate (contrary to intuition) will be
poorer in ethanol than the original but slightly richer than the
azeotrope. This means the solution left behind will be richer in ethanol.
If two
solvents can form a negative azeotrope, then distillation of any mixture of
those constituents will result in the residue being closer in
composition to the azeotrope than the original mixture. For example, if
a hydrochloric acid solution contains less than 20.2% hydrogen
chloride, boiling the mixture will leave behind a solution that is richer in
hydrogen chloride than the original. If the solution initially contains more
than 20.2% hydrogen chloride, then boiling will leave behind a solution that is
poorer in hydrogen chloride than the original. Boiling of any hydrochloric acid
solution long enough will cause the solution left behind to approach the
azeotropic ratio.
Phase diagrams
The boiling
and recondensation of a mixture of two solvents are changes of state. As such,
they are best illustrated with a phase diagram. If pressure is held constant,
the two parameters that can vary are the temperature and the composition. An
azeotrope is not the same as an emulsion.
Condition of existence
The
condition of existence follows from the equality of pressures in vapour-liquid
equilibrium using Raoult and Dalton laws for real mixtures and imposing the
equality of compositions in liquid and vapour phases.
The
condition relates activity coefficients in liquid phase to total pressure and
the vapour pressures of pure components.
Minimum-boiling or Positive azeotrope
The diagram
on the right shows a positive azeotrope of hypothetical constituents, X and Y.
The bottom trace illustrates the boiling temperature of various compositions.
Below the bottom trace, only the liquid phase is in equilibrium. The top trace
illustrates the vapor composition above the liquid at a given temperature.
Above the top trace, only the vapor is in equilibrium. Between the two traces,
liquid and vapor phases exist simultaneously in equilibrium: for example,
heating a 25% X : 75% Y mixture to temperature AB would generate vapor of
composition B over liquid of composition A.
The azeotrope is the point on the
diagram where the two curves touch. The horizontal and vertical steps show the
path of repeated distillations. Point A is the boiling point of a nonazeotropic
mixture.
The vapor that separates at that temperature has composition B. The
shape of the curves requires that the vapor at B be richer in constituent X
than the liquid at point A. The vapor is physically separated from the VLE
(vapor-liquid equilibrium) system and is cooled to point C, where it condenses.
The resulting liquid (point C) is now richer in X than it was at point A.
If
the collected liquid is boiled again, it progresses to point D, and so on. The
stepwise progression shows how repeated distillation can never produce a
distillate that is richer in constituent X than the azeotrope. Note that
starting to the right of the azeotrope point results in the same stepwise
process closing in on the azeotrope point from the other
direction.
Maximum-boiling or Negative azeotrope
The diagram
on the right shows a negative azeotrope of ideal constituents, X and Y. Again
the bottom trace illustrates the boiling temperature at various compositions,
and again, below the bottom trace the mixture must be entirely liquid phase.
The top trace again illustrates the condensation temperature of various
compositions, and again, above the top trace the mixture must be entirely vapor
phase. The point, A, shown here is a boiling point with a composition chosen
very near to the azeotrope. The vapor is collected at the same temperature at
point B. That vapor is cooled, condensed, and collected at point C. Because
this example is a negative azeotrope rather than a positive one, the distillate
is farther from the azeotrope than the original liquid mixture at point A was.
So the distillate is poorer in constituent X and richer in constituent Y than
the original mixture. Because this process has removed a greater fraction of Y
from the liquid than it had originally, the residue must be poorer in Y and
richer in X after distillation than before.
If the
point, A had been chosen to the right of the azeotrope rather than to the left,
the distillate at point C would be farther to the right than A, which is to say
that the distillate would be richer in X and poorer in Y than the original
mixture. So in this case too, the distillate moves away from the azeotrope and
the residue moves toward it. This is characteristic of negative azeotropes. No
amount of distillation, however, can make either the distillate or the residue
arrive on the opposite side of the azeotrope from the original mixture. This is
characteristic of all azeotropes.
Traces
The traces
in the phase diagrams separate whenever the composition of the vapor differs
from the composition of the liquid at the same temperature. Suppose the total
composition were 50/50%. You could make this composition using 50% of 50/50%
vapor and 50% of 50/50% liquid, but you could also make it from 83.33% of
45/55% vapor and 16.67% of 75%/25% liquid, as well as from many other
combinations. The separation of the two traces represents the range of
combinations of liquid and vapor that can make each total composition.
Alternatively,
one can view the lower trace as the boundary for the region of the diagram in
which liquids are in equilibrium, and the upper trace as the boundary of the
region in which the vapor is in equilibrium. These two boundaries need not
coincide. Indeed, the region between them is a no-man's-land: attempts to bring
the system to the midpoint of line-segment AB will result in a mixture of
liquid A and vapor B, but nothing at the midpoint.
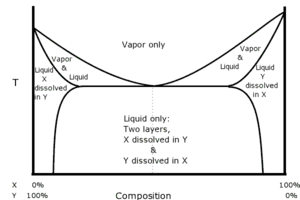
Heteroazeotropes
In each of
the examples discussed so far the constituents have been miscible in all
proportions with each other. For example, any amount of ethanol can be mixed
with any amount of water to form a homogeneous solution. There are pairs of
solvents for which this is not the case. For example, if equal volumes of
chloroform (water solubility 0.8 g/100 ml at 20 °C) and water are shaken
together and then left to stand, the liquid will separate into two layers.
Analysis of the layers shows that the top layer is mostly water with a small
amount of chloroform dissolved in it, and the bottom layer is mostly chloroform
with a small amount of water dissolved in it. If the two layers are heated
together, the system of layers will boil at 53.3 °C, which is lower than either
the boiling point of chloroform (61.2 °C) or the boiling point of water (100
°C). The vapor will consist of 97.0% chloroform and 3.0% water regardless of
how much of each liquid layer is present (provided both layers are indeed
present). If the vapor is re-condensed, the layers will reform in the
condensate, and will do so in a fixed ratio, which in this case is 4.4% of the
volume in the top layer and 95.6% in the bottom layer.[11] Such a system of
solvents is known as a heteroazeotrope. The diagram illustrates how the various
phases of a heteroazeotrope are related.
Heteroazeotropes
are always minimum boiling mixtures.
Deviation from Raoult's law
Raoult's law
predicts the vapor pressures of ideal mixtures as a function of composition
ratio. In general only mixtures of chemically similar solvents, such as
n-hexane with n-heptane, form nearly ideal mixtures that come close to obeying
Raoult's law. Solvent combinations that can form azeotropes are always
nonideal, and as such they deviate from Raoult's law.
The diagram
on the right illustrates total vapor pressure of three hypothetical mixtures of
constituents, X, and Y. The temperature throughout the plot is assumed to be
constant.
The center
trace is a straight line, which is what Raoult's law predicts for an ideal
mixture. The top trace illustrates a nonideal mixture that has a positive
deviation from Raoult's law, where the total combined vapor pressure of
constituents, X and Y, is greater than what is predicted by Raoult's law. The
top trace deviates sufficiently that there is a point on the curve where its
tangent is horizontal. Whenever a mixture has a positive deviation and has a
point at which the tangent is horizontal, the composition at that point is a
positive azeotrope.At that point the total vapor pressure is at a maximum.
Likewise the bottom trace illustrates a nonideal mixture that has a negative
deviation from Raoult's law, and at the composition where tangent to the trace
is horizontal there is a negative azeotrope. This is also the point where total
vapor pressure is minimum.
Temperature-pressure dependence
For both the
top and bottom traces, the temperature point of the azeotrope is the constant
temperature chosen for the graph. If the ambient pressure is controlled to be
equal to the total vapor pressure at the azeotropic mixture, then the mixture
will boil at this fixed temperature.
Vapor
pressure of both pure liquids as well as mixtures is a sensitive function of
temperature. As a rule, vapor pressure of a liquid increases nearly
exponentially as a function of temperature. If the graph were replotted for a
different fixed temperature, then the total vapor pressure at the azeotropic
composition will certainly change, but it is also possible that the composition
at which the azeotrope occurs will change. This implies that the composition of
an azeotrope is affected by the pressure chosen at which to boil the mixture.
Ordinarily distillation is done at atmospheric pressure, but with proper
equipment it is possible to carry out distillation at a wide variety of pressures,
both above and below atmospheric pressure.
Separation of constituents
Distillation
is one of the primary tools that chemists and chemical engineers use to
separate mixtures into their constituents. Because distillation cannot separate
the constituents of an azeotrope, the separation of azeotropic mixtures (also
called azeotrope breaking) is a topic of considerable interest. Indeed, this
difficulty led some early investigators to believe that azeotropes were
actually compounds of their constituents.[1] But there are two reasons for
believing that this is not the case. One is that the molar ratio of the
constituents of an azeotrope is not generally the ratio of small integers. For
example, the azeotrope formed by water and acetonitrile contains 2.253 moles (
or 9/4 with a relative error of just 2% ) of acetonitrile for each mole of
water.[13] A more compelling reason for believing that azeotropes are not
compounds is, as discussed in the last section, that the composition of an
azeotrope can be affected by pressure. Contrast that with a true compound,
carbon dioxide for example, which is two moles of oxygen for each mole of
carbon no matter what pressure the gas is observed at. That azeotropic
composition can be affected by pressure suggests a means by which such a
mixture can be separated.
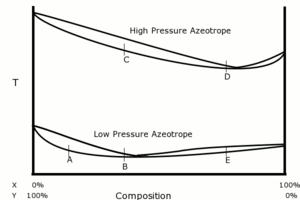
Pressure swing distillation
A
hypothetical azeotrope of constituents X and Y is shown in the adjacent
diagram. Two at an arbitrarily chosen low pressure and another at an
arbitrarily chosen, but higher, pressure. The composition of the azeotrope is
substantially different between the high- and low-pressure plots – higher in X
for the high-pressure system. The goal is to separate X in as high a
concentration as possible starting from point A. At the low
pressure, it is possible by progressive distillation to reach a distillate at
the point, B, which is on the same side of the azeotrope as A.
Note that successive distillation steps near the azeotropic composition exhibit
very little difference in boiling temperature. If this distillate is now
exposed to the high pressure, it boils at point C. From C,
by progressive distillation it is possible to reach a distillate at the
point D, which is on the same side of the high-pressure azeotrope
as C. If that distillate is then exposed again to the low pressure,
it boils at point E, which is on the opposite side
of the low-pressure azeotrope to A. So, by means of the pressure
swing, it is possible to cross over the low-pressure azeotrope.
When the
solution is boiled at point E, the distillate is poorer in X than
the residue at point E. This means that the residue is richer in X
than the distillate at point E. Indeed, progressive distillation
can produce a residue as rich in X as is required.
In summary:
1.
Low-pressure rectification (A to B)
2. High-pressure rectification (C to D) 3. Low-pressure stripping (E to target purity) |
|
Note that
both azeotropes above are of the positive, or minimum
boiling type; care must be taken to ensure that the correct component
of the separation step is retained, i.e. the binary phase-envelope diagram (boiling-point curve) must be correctly
read.
A mixture of
5% water with 95% tetrahydrofuran is an example of an azeotrope that
can be economically separated using a pressure swing – a swing in this case
between 1 atm and 8 atm. By contrast the
composition of the water to ethanol azeotrope discussed earlier is not affected
enough by pressure to be easily separated using pressure swings and
instead, an entrainer may be added that either modifies the
azeotropic composition and exhibits immiscibility with one of the
components, or extractive distillation may be used.
Azeotropic distillation
Other
methods of separation involve introducing an additional agent, called an
entrainer, that will affect the volatility of one of the azeotrope constituents
more than another. When an entrainer is added to a binary azeotrope to form a
ternary azeotrope, and the resulting mixture distilled, the method is called
azeotropic distillation. The best known example is adding benzene or cyclohexane
to the water/ethanol azeotrope. With cyclohexane as the entrainer, the ternary
azeotrope is 7% water, 17% ethanol, and 76% cyclohexane, and boils at 62.1 °C.
Just enough cyclohexane is added to the water/ethanol azeotrope to engage all
of the water into the ternary azeotrope. When the mixture is then boiled, the
azeotrope vaporizes leaving a residue composed almost entirely of the excess
ethanol.
Chemical action separation
Another type
of entrainer is one that has a strong chemical affinity for one of the
constituents. Using again the example of the water/ethanol azeotrope, the
liquid can be shaken with calcium oxide, which reacts strongly with water to
form the nonvolatile compound, calcium hydroxide. Nearly all of the calcium
hydroxide can be separated by filtration and the filtrate redistilled to obtain
100% pure ethanol.
A more
extreme example is the azeotrope of 1.2% water with 98.8% diethyl ether. Ether
holds the last bit of water so tenaciously that only a very powerful desiccant
such as sodium metal added to the liquid phase can result in completely dry
ether.
Anhydrous
calcium chloride is used as a desiccant for drying a wide variety of solvents
since it is inexpensive and does not react with most nonaqueous solvents.
Chloroform is an example of a solvent that can be effectively dried using
calcium chloride.
Distillation using a dissolved salt
When a salt
is dissolved in a solvent, it always has the effect of raising the boiling
point of that solvent – that is it decreases the volatility of the solvent.
When the salt is readily soluble in one constituent of a mixture but not in
another, the volatility of the constituent in which it is soluble is decreased
and the other constituent is unaffected. In this way, for example, it is
possible to break the water/ethanol azeotrope by dissolving potassium acetate
in it and distilling the result.
Extractive distillation
Extractive
distillation is similar to azeotropic distillation, except in this case the
entrainer is less volatile than any of the azeotrope's constituents. For
example, the azeotrope of 20% acetone with 80% chloroform can be broken by
adding water and distilling the result. The water forms a separate layer in
which the acetone preferentially dissolves. The result is that the distillate
is richer in chloroform than the original azeotrope.
Pervaporation and other membrane methods
The
pervaporation method uses a membrane that is more permeable to the one
constituent than to another to separate the constituents of an azeotrope as it
passes from liquid to vapor phase. The membrane is rigged to lie between the
liquid and vapor phases. Another membrane method is vapor permeation, where the
constituents pass through the membrane entirely in the vapor phase. In all
membrane methods, the membrane separates the fluid passing through it into a
permeate (that which passes through) and a retentate (that which is left
behind). When the membrane is chosen so that is it more permeable to one
constituent than another, then the permeate will be richer in that first
constituent than the retentate.
Zeotropic mixtures
Sometimes
azeotropes are useful in separating zeotropic mixtures. An example is acetic
acid and water, which do not form an azeotrope. Despite this it is very
difficult to separate pure acetic acid (boiling point: 118.1 °C) from a
solution of acetic acid and water by distillation alone. As progressive
distillations produce solutions with less and less water, each further
distillation becomes less effective at removing the remaining water. Distilling
the solution to dry acetic acid is therefore economically impractical. But
ethyl acetate forms an azeotrope with water that boils at 70.4 °C. By adding
ethyl acetate as an entrainer, it is possible to distill away the azeotrope and
leave nearly pure acetic acid as the residue.
Mechanism
Azeotropes
can only form when a mixture deviates from Raoult's law. Raoult's law applies
when the molecules of the constituents stick to each other to the same degree
as they do to themselves. For example, if the constituents are X and Y, then X
sticks to Y with roughly equal energy as X does with X and Y does with Y. A
positive deviation from Raoult's law results when the constituents have a
disaffinity for each other – that is X sticks to X and Y to Y better than X
sticks to Y. Because this results in the mixture having less total sticking
together of the molecules than the pure constituents, they more readily escape
from the stuck-together phase, which is to say the liquid phase, and into the
vapor phase. When X sticks to Y more aggressively than X does to X and Y does
to Y, the result is a negative deviation from Raoult's law. In this case
because there is more sticking together of the molecules in the mixture than in
the pure constituents, they are more reluctant to escape the stuck-together
liquid phase.
When the
deviation is great enough to cause a maximum or minimum in the vapor pressure
versus composition function, it is a mathematical consequence that at that
point, the vapor will have the same composition as the liquid, and so an
azeotrope is the result.
Complex systems
The rules
for positive and negative azeotropes apply to all the examples discussed so
far. But there are some examples that don't fit into the categories of positive
or negative azeotropes. The best known of these is the ternary azeotrope formed
by 30% acetone, 47% chloroform, and 23% methanol, which boils at 57.5 °C. Each
pair of these constituents forms a binary azeotrope, but chloroform/methanol
and acetone/methanol both form positive azeotropes while chloroform/acetone
forms a negative azeotrope. The resulting ternary azeotrope is neither positive
nor negative. Its boiling point falls between the boiling points of acetone and
chloroform, so it is neither a maximum nor a minimum boiling point. This type
of system is called a saddle azeotrope.Only systems of three or more
constituents can form saddle azeotropes.
A rare type
of complex binary azeotrope is one where the boiling point and condensation
point curves touch at two points in the phase diagram. Such a system is called
a double azeotrope, and will have two azeotropic compositions and boiling
points. An example is water and N-methylethylenediamine.
















No comments