Periodic gradation of the elements in the third period - PERIODIC CHEMISTRY
The period
3 element is one of the chemicalelements in the third row (or period) of the periodic
table of the chemical elements. The periodic table is laid out in
rows to
illustrate recurring (periodic) trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when the periodic table skips a row and a chemical behaviour begins to repeat, meaning that elements with similar behavior fall into the same vertical columns. The third period contains eight elements: sodium, magnesium, aluminium, silicon, phosphorus, sulfur, chlorine, and argon. The first two, sodium and magnesium, are members of the s-block of the periodic table, while the others are members of the p-block. Note that there is a 3d subshell, but it is not filled until period4, such giving the periodic table its characteristic shape of "two rows at a time". All of the period 3 elements occur in nature and have at least one stable isotope.[1]
illustrate recurring (periodic) trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when the periodic table skips a row and a chemical behaviour begins to repeat, meaning that elements with similar behavior fall into the same vertical columns. The third period contains eight elements: sodium, magnesium, aluminium, silicon, phosphorus, sulfur, chlorine, and argon. The first two, sodium and magnesium, are members of the s-block of the periodic table, while the others are members of the p-block. Note that there is a 3d subshell, but it is not filled until period4, such giving the periodic table its characteristic shape of "two rows at a time". All of the period 3 elements occur in nature and have at least one stable isotope.[1]
Periodic trends[edit]
Atomic radius
As the atomic number of elements in Period 3 increases, the atomic radius decreases.
Electronegativity
As the atomic mass (A) of elements in Period 3 increases, the electronegativity increases.
Ionization energy
As the atomic number of elements in Period 3 increases, the amount of energy required to remove its electrons (Ionization energy) increases.
Elements
Chemical element Chemical series Electron configuration 11 Na Sodium Alkali metal [Ne] 3s1 12 Mg Magnesium Alkaline earth metal [Ne] 3s2 13 Al Aluminium Post-transition metal [Ne] 3s2 3p1 14 Si Silicon Metalloid [Ne] 3s2 3p2 15 P Phosphorus Polyatomic nonmetal [Ne] 3s2 3p3 16 S Sulfur Polyatomic nonmetal [Ne] 3s2 3p4 17 Cl Chlorine Diatomic nonmetal [Ne] 3s2 3p5 18 Ar Argon Noble gas [Ne] 3s2 3p6
Sodium
Sodium (symbol Na) is a soft, silvery-white, highly reactive metal and
is a member of the alkali metals; its only stable isotope is 23Na. It is an
abundant element that exists in numerous minerals such as feldspars, sodalite
and rock salt. Many salts of sodium are highly soluble in water and are thus
present in significant quantities in the Earth's bodies of water, most
abundantly in the oceans as sodium chloride.
Many sodium compounds are useful, such as sodium hydroxide (lye) for
soapmaking, and sodium chloride for use as a deicing agent and a nutrient.
The free metal, elemental sodium, does not occur in nature but must be
prepared from sodium compounds. Elemental sodium was first isolated by Humphry
Davy in 1807 by the electrolysis of sodium hydroxide. The same ion is also a
component of many minerals, such as sodium nitrate.
Magnesium
Magnesium (symbol Mg) is an alkaline earth metal and has common
oxidation number +2. It is the eighth most abundant element in the Earth's
crust and ninth in the known universe as a whole. Magnesium is the fourth most
common element in the Earth as a whole (behind iron, oxygen and silicon),
making up 13% of the planet's mass and a large fraction of planet's mantle. The
relative abundance of magnesium is related to the fact that it is easily built
up in supernova stars from a sequential addition of three helium nuclei to
carbon (which in turn is made from three helium nuclei). Due to magnesium ion's
high solubility in water, it is the third most abundant element dissolved in
seawater.
The free element (metal) is not found naturally on Earth, as it is
highly reactive (though once produced, it is coated in a thin layer of oxide
[see passivation], which partly masks this reactivity). The free metal burns
with a characteristic brilliant white light, making it a useful ingredient in
flares. The metal is now mainly obtained by electrolysis of magnesium salts
obtained from brine. Commercially, the chief use for the metal is as an
alloying agent to make aluminium-magnesium alloys, sometimes called
"magnalium" or "magnelium". Since magnesium is less dense
than aluminium, these alloys are prized for their relative lightness and
strength.
Magnesium ions are sour to the taste, and in low concentrations help to
impart a natural tartness to fresh mineral waters.
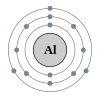
Aluminium
Aluminium (symbol Al) or aluminum (American English) is a silvery white
member of the boron group of chemical elements and a post-transition metal. It
is not soluble in water under normal circumstances. Aluminium is the third most
abundant element (after oxygen and silicon), and the most abundant metal, in
the Earth's crust. It makes up about 8% by weight of the Earth's solid surface.
Aluminium metal is too reactive chemically to occur natively. Instead, it is
found combined in over 270 different minerals.[6] The chief ore of aluminium is
bauxite.
Aluminium is remarkable for the metal's low density and for its ability
to resist corrosion due to the phenomenon of passivation. Structural components
made from aluminium and its alloys are vital to the aerospace industry and are
important in other areas of transportation and structural materials. The most
useful compounds of aluminium, at least on a weight basis, are the oxides and
sulfates.
Silicon
Silicon (symbol Si) is a tetravalent metalloid. It is less reactive
than its chemical analog carbon, the nonmetal directly above it in the periodic
table, but more reactive than germanium, the metalloid directly below it in the
table. Controversy about silicon's character dates to its discovery: silicon
was first prepared and characterized in pure form in 1824, and given the name
silicium (from Latin: silicis, flints), with an -ium word-ending to suggest a
metal. However, its final name, suggested in 1831, reflects the more physically
similar elements carbon and boron.
Silicon is the eighth most common element in the universe by mass, but
very rarely occurs as the pure free element in nature. It is most widely
distributed in dusts, sands, planetoids, and planets as various forms of
silicon dioxide (silica) or silicates. Over 90% of the Earth's crust is
composed of silicate minerals, making silicon the second most abundant element
in the earth's crust (about 28% by mass) after oxygen.
Most silicon is used commercially without being separated, and indeed
often with little processing of compounds from nature. These include direct
industrial building-use of clays, silica sand and stone. Silica is used in
ceramic brick. Silicate goes into Portland cement for mortar and stucco, and
when combined with silica sand and gravel, to make concrete. Silicates are also
in whiteware ceramics such as porcelain, and in traditional quartz-based
soda-lime glass. More modern silicon compounds such as silicon carbide form
abrasives and high-strength ceramics. Silicon is the basis of the ubiquitous
synthetic silicon-based polymers called silicones.
Elemental silicon also has a large impact on the modern world economy.
Although most free silicon is used in the steel refining, aluminum-casting, and
fine chemical industries (often to make fumed silica), the relatively small
portion of very highly purified silicon that is used in semiconductor
electronics (< 10%) is perhaps even more critical. Because of wide use of
silicon in integrated circuits, the basis of most computers, a great deal of
modern technology depends on it.
Phosphorus
Phosphorus (symbol P) is a multivalent nonmetal of the nitrogen group,
phosphorus as a mineral is almost always present in its maximally oxidized
state, as inorganic phosphate rocks. Elemental phosphorus exists in two major
forms—white phosphorus and red phosphorus—but due to its high reactivity,
phosphorus is never found as a free element on Earth.
The first form of elemental phosphorus to be produced (white
phosphorus, in 1669) emits a faint glow upon exposure to oxygen – hence its
name given from Greek mythology, Φωσφόρος meaning "light-bearer"
(Latin Lucifer), referring to the "Morning Star", the planet Venus.
Although the term "phosphorescence", meaning glow after illumination,
derives from this property of phosphorus, the glow of phosphorus originates
from oxidation of the white (but not red) phosphorus and should be called
chemiluminescence. It is also the lightest element to easily produce stable
exceptions to the octet rule.
The vast majority of phosphorus compounds are consumed as fertilizers.
Other applications include the role of organophosphorus compounds in
detergents, pesticides and nerve agents, and matches.[8]
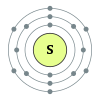
Sulfur
Sulfur (symbol S) is an abundant, multivalentnon-metal. Under normal
conditions, sulfur atoms form cyclic octatomic molecules with chemical formula
S8. Elemental sulfur is a bright yellow crystalline solid when at room
temperature. Chemically, sulfur can react as either an oxidant or reducing
agent. It oxidizes most metals and several nonmetals, including carbon, which
leads to its negative charge in most organosulfur compounds, but it reduces several
strong oxidants, such as oxygen and fluorine.
In nature, sulfur can be found as the pure element and as sulfide and
sulfate minerals. Elemental sulfur crystals are commonly sought after by
mineral collectors for their brightly colored polyhedron shapes. Being abundant
in native form, sulfur was known in ancient times, mentioned for its uses in
ancient Greece, China and Egypt. Sulfur fumes were used as fumigants, and
sulfur-containing medicinal mixtures were used as balms and antiparasitics.
Sulfur is referenced in the Bible as brimstone in English, with this name still
used in several nonscientific terms.[9] Sulfur was considered important enough
to receive its own alchemical symbol. It was needed to make the best quality of
black gunpowder, and the bright yellow powder was hypothesized by alchemists to
contain some of the properties of gold, which they sought to synthesize from
it. In 1777, Antoine Lavoisier helped convince the scientific community that
sulfur was a basic element, rather than a compound.
Elemental sulfur was once extracted from salt domes where it sometimes
occurs in nearly pure form, but this method has been obsolete since the late
20th century. Today, almost all elemental sulfur is produced as a byproduct of
removing sulfur-containing contaminants from natural gas and petroleum. The
element's commercial uses are primarily in fertilizers, because of the
relatively high requirement of plants for it, and in the manufacture of
sulfuric acid, a primary industrial chemical. Other well-known uses for the
element are in matches, insecticides and fungicides. Many sulfur compounds are
odiferous, and the smell of odorized natural gas, skunk scent, grapefruit, and
garlic is due to sulfur compounds. Hydrogen sulfide produced by living
organisms imparts the characteristic odor to rotting eggs and other biological
processes.
Chlorine
Chlorine (symbol Cl) is the second lightest halogen, found in the
periodic table in group 17. The element forms diatomic molecules under standard
conditions, called dichlorine. It has the highest electron affinity and the
third highest electronegativity of all the elements; for this reason, chlorine
is a strong oxidizing agent.
The most common compound of chlorine, sodium chloride, has been known
since ancient times; however, around 1630, chlorine gas was obtained by the
Belgian chemist and physician Jan Baptist van Helmont. The synthesis and
characterization of elemental chlorine occurred in 1774 by Swedish chemist Carl
Wilhelm Scheele, who called it "dephlogisticated muriatic acid air,"
having thought he synthesized the oxide obtained from the hydrochloric acid,
because acids were thought at the time to necessarily contain oxygen, a number
of chemists, including Claude Berthollet, suggested that Scheele's
dephlogisticated muriatic acid air must be a combination of oxygen and the yet
undiscovered element, and Scheele named the supposed new element within this
oxide as muriaticum. The suggestion that this newly discovered gas was a simple
element was made in 1809 by Joseph Louis Gay-Lussac and Louis-Jacques. This was
confirmed by Sir Humphry Davy in 1810, who named it chlorine, from the Greek
word χλωρος (chlōros), meaning "green-yellow."
Chlorine is a component of various compounds, including table salt. It
is the second most abundant halogen and 21st most abundant chemical element in
Earth's crust. The great oxidizing potential of chlorine led it to its
bleaching and disinfectant uses, as well as uses of an essential reagent in the
chemical industry. As a common disinfectant, chlorine compounds are used in
swimming pools to keep them clean and sanitary. In the upper atmosphere,
chlorine-containing molecules such as chlorofluorocarbons have been implicated
in ozone depletion.
Argon
Argon (symbol Ar) is the third element in group 18 of the periodic
table (the noble gases). Argon is the third most common gas in the Earth's
atmosphere, at 0.93%, making it more common than carbon dioxide. Nearly all of
this argon is radiogenic argon-40 derived from the decay of potassium-40 in the
Earth's crust. In the universe, argon-36 is by far the most common argon
isotope, being the preferred argon isotope produced by stellar nucleosynthesis
in supernovas.
The name "argon" is derived from the Greek word αργον meaning
"lazy" or "the inactive one", a reference to the fact that
the element undergoes almost no chemical reactions. The complete octet (eight
electrons) in the outer atomic shell makes argon stable and resistant to
bonding with other elements. Its triple point temperature of 83.8058 K is a
defining fixed point in the International Temperature Scale of 1990.
Argon is produced industrially by the fractional distillation of liquid
air. Argon is mostly used as an inert shielding gas in welding and other high-temperature
industrial processes where ordinarily non-reactive substances become reactive;
for example, an argon atmosphere is used in graphite electric furnaces to
prevent the graphite from burning. Argon gas also has uses in incandescent and
fluorescent lighting, and other types of gas discharge tubes. Argon makes a
distinctive blue-green gas laser.
READ THE 2017/2018 Syllabus
READ THE 2017/2018 Syllabus

















You may search for our products through the search bar on our website. If you would like to receive a copy of our product catalog, please contact us at info@alfa-chemistry.com. Liq
ReplyDelete