Chemistry News:Transition metal–catalyzed alkyl-alkyl bond formation - Another Great dimension in cross-coupling chemistry
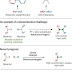
Alkyl-alkyl
bond formation, including control of stereochemistry: an ongoing challenge in
organic synthesis.
From top to
bottom: sp2- versus sp3-hybridized carbon-carbon bonds;
the difficulty of stereochemical control; and enantioconvergent reactions of
racemic secondary electrophiles and racemic nucleophiles. X, leaving group; M,
metal.
Because the backbone of most organic molecules is composed primarily of
carbon-carbon bonds, the development of efficient methods for their
construction is one of the central challenges of organic synthesis. Transition
metal–catalyzed cross-coupling
reactions between organic electrophiles and
nucleophiles serve as particularly powerful tools for achieving carbon-carbon
bond formation. Until recently, the vast majority of cross-coupling processes
had used either aryl or alkenyl electrophiles as one of the coupling partners.
In the past 15 years, versatile new methods have been developed that effect
cross-couplings of an array of alkyl electrophiles, thereby greatly expanding
the diversity of target molecules that are readily accessible. The ability to
couple alkyl electrophiles opens the door to a stereochemical
dimension—specifically, enantioconvergent couplings of racemic
electrophiles—that substantially enhances the already remarkable utility of
cross-coupling processes.
Stitching
one alkyl group to another
Chemical reactions such as Heck and Suzuki coupling facilitate access to
an enormous range of relatively flat molecules. This geometrical constraint is
associated with the comparative ease of linking together aryl and alkenyl
carbons. Choi and Fu review recent developments in forming bonds between the
more abundant alkyl carbon centers that underlie diverse molecules with complex
three-dimensional structures. Nickel catalysis in particular has emerged as a
powerful method to access individual mirror-image isomers selectively and
thereby tune the biological properties of the targeted products.
Science, this issue p. eaaf7230
Structured
Abstract
BACKGROUND
The development of useful new methods for the construction of
carbon-carbon bonds has had an impact on the many scientific disciplines
(including materials science, biology, and chemistry) that use organic
compounds. Tremendous progress has been made in the past several decades in the
creation of bonds between sp2-hybridized carbons (e.g., aryl-aryl
bonds), particularly through the use of transition metal catalysis. In
contrast, until recently, advances in the development of general methods that
form bonds between sp3-hybridized carbons (alkyl-alkyl bonds) had
been rather limited. A variety of approaches, such as classical SN2
reactions and transition metal catalysis, typically led to side reactions
rather than the desired carbon-carbon bond formation. With transition metal
catalysis, the unwanted but often facile β-hydride elimination of alkylmetal
complexes presented a key impediment to efficient cross-coupling of alkyl
electrophiles.
In the case of many alkyl-alkyl bonds, there is an additional challenge
beyond construction of the carbon-carbon bond itself: controlling the
stereochemistry at one or both carbons of the new bond. It is important to
control the stereochemistry of organic molecules because of its influence on
properties such as biological activity.
Each of these two challenges is difficult to solve individually;
addressing them simultaneously is even more demanding. Until recently, the
methods for achieving alkyl-alkyl bond formation were comparatively limited in
scope, typically involving the use of unhindered (e.g., primary) electrophiles
and unhindered, highly reactive nucleophiles (e.g., Grignard reagents, which
have relatively poor functional group compatibility). With respect to
enantioconvergent reactions, there were virtually no examples.
ADVANCES
In recent years, it has been established that, through the action of an
appropriate transition metal catalyst, it is possible to achieve a broad range
of alkyl-alkyl bond-forming processes; nickel-based catalysts have proved to be
especially effective. With respect to the electrophilic coupling partner, a
wide range of secondary alkyl halides are now suitable. This has enabled the
development of enantioconvergent reactions of readily available racemic
secondary electrophiles. In view of the abundance of tertiary stereocenters in
organic molecules, this is a noteworthy advance in synthesis.
With respect to the nucleophilic partner, alkylboron and alkylzinc
reagents (Suzuki- and Negishi-type reactions, respectively) can now be used in
a wide variety of alkyl-alkyl couplings, which greatly increases the utility of
such processes, as these nucleophiles are more readily available and have much
improved functional group compatibility relative to Grignard reagents. These
new methods for alkyl-alkyl bond formation have been applied to the synthesis
of natural products and other bioactive compounds.
OUTLOOK
A number of major challenges remain. For example, with regard to the
electrophilic coupling partner, there is a need to develop general methods that
are effective for tertiary alkyl halides, including enantioconvergent
processes. With regard to the nucleophilic partner, there is a need to discover
more versatile catalysts that can use a wide range of hindered (e.g., secondary
and tertiary) alkylmetal reagents, as well as to achieve a broad spectrum of
enantioconvergent couplings of racemic nucleophiles. These advances can enable
the doubly stereoconvergent coupling of a racemic electrophile with a racemic
nucleophile.
The synthesis of alkyl-alkyl bonds is arguably the most important bond
construction in organic synthesis. The ability to achieve this bond formation
at will, as well as to control the product stereochemistry, would transform
organic synthesis and empower the many scientists who use organic molecules.
Recent work has provided evidence that transition metal catalysis can address
this exciting challenge.
Continue at the original source










No comments