Carbon And Its Properties... All about The "king of the elements"
Elemental Carbon (from Latin: carbo "coal") is a chemical element with
symbol C and atomic number 6.
It is nonmetallic and tetravalent element—making four
electrons available to form covalent chemical bonds.
Three of its isotopes occur
naturally, which Carbon 12 and Carbon13 being stable, while 14C is a radioactive isotope,
decaying with a half-life of about 5,730 years.
Carbon is one of the few
elements known since antiquity. It is the 15th most abundant element in the Earth's crust, and the
fourth most abundant element in the universe by mass after hydrogen, helium,
and oxygen.
Carbon's abundance, its unique diversity of organic compounds, and
its unusual ability to form polymers at the temperatures commonly encountered
on Earth enables this element to serve as a common element of all known life.
It is the second most abundant element in the human body by mass (about 18.5%)
after oxygen.
The atoms of carbon can bond together in different ways, calls allotropes of carbon. The best known are graphite, diamond, and amorphous
carbon.
The physical properties of carbon vary widely with the allotropic
form.
For example, graphite is opaque and black while diamond is highly
transparent.
Graphite is soft enough to form a streak on paper (hence its name,
from the Greek verb "γράφειν" which means "to write" that is why it is used in the manufacturing of pencil ),
while diamond is the hardest naturally occurring material known.
Graphite is a
good electrical conductor while diamond has a low electrical conductor.
Under normal conditions, diamond, carbon nanotubes, and graphene have the
highest thermal conductivities of all known materials.
All carbon allotropes are
solids under normal conditions, with graphite being the most thermodynamically
stable form.
They are chemically resistant and require high temperature to
react even with oxygen.
The most common oxidation state of carbon in inorganic compounds is +4,
while +2 is found in carbon monoxide and transition metal carbonyl complexes.
The largest sources of inorganic carbon are limestones, dolomites and carbon
dioxide, but significant quantities occur in organic deposits of coal, peat,
oil, and methane clathrates.
Carbon forms a vast number of compounds, more than
any other element, with almost ten million compounds described to date, and
yet that number is but a fraction of the number of theoretically possible
compounds under standard conditions.
For this reason, carbon has often been
referred to as the "king of the elements".
Characteristics
The allotropes of carbon include graphite, one of the softest known
substances, and diamond, the hardest naturally occurring substance. It bonds
readily with other small atoms including other carbon atoms, and is capable of
forming multiple stable covalent bonds with suitable, multivalent atoms.
Carbon
is known to form almost ten million different compounds, a large majority of
all chemical compounds.
Carbon also has the highest sublimation point of
all elements.
At atmospheric pressure it has no melting point as its triple
point is at 10.8 ± 0.2 MPa and 4,600 ± 300 K (~4,330 °C or 7,820 °F), so
it sublimes at about 3,900 K.
Graphite is much more reactive than
diamond at standard conditions, despite being more thermodynamically stable, as
its delocalised pi system is much more vulnerable to attack. For example,
graphite can be oxidised by hot concentrated nitric acid at standard conditions
to mellitic acid, C6(CO2H)6, which preserves the hexagonal units of graphite
while breaking up the larger structure.
Carbon sublimes in a carbon arc which has a temperature of about 5,800 K
(5,530 °C; 9,980 °F).
Thus, irrespective of its allotropic form, carbon remains
solid at higher temperatures than the highest melting point metals such as
tungsten or rhenium. Although thermodynamically prone to oxidation, carbon
resists oxidation more effectively than elements such as iron and copper that
are weaker reducing agents at room temperature.
Carbon is the sixth element, with a ground-state electron configuration
of 1s22s22p2, of which the four outer electrons are valence electrons. Its
first four ionisation energies, 1086.5, 2352.6, 4620.5 and 6222.7 kJ/mol, are
much higher than those of the heavier group 14 elements. The electronegativity
of carbon is 2.5, significantly higher than the heavier group 14 elements
(1.8–1.9), but close to most of the nearby nonmetals as well as some of the
second- and third-row transition metals. Carbon's covalent radii are normally
taken as 77.2 pm (C–C), 66.7 pm (C=C) and 60.3 pm (C≡C), although these may
vary depending on coordination number and what the carbon is bonded to. In
general, covalent radius decreases with lower coordination number and higher
bond order.[24]
Carbon compounds form the basis of all known life on Earth, and the
carbon-nitrogen cycle provides some of the energy produced by the Sun and other
stars. Although it forms an extraordinary variety of compounds, most forms of
carbon are comparatively unreactive under normal conditions. At standard
temperature and pressure, it resists all but the strongest oxidizers. It does
not react with sulfuric acid, hydrochloric acid, chlorine or any alkalis.
At
elevated temperatures, carbon reacts with oxygen to form carbon oxides, and
will rob oxygen from metal oxides to leave the elemental metal. This exothermic
reaction is used in the iron and steel industry to smelt iron and to control
the carbon content of steel:
Fe3O4 + 4 C(s) → 3 Fe(s) + 4 CO(g)
with sulfur to form carbon disulfide and with steam in the coal-gas
reaction:
C(s) + H2O(g) → CO(g) + H2(g).
Carbon combines with some metals at high temperatures to form metallic
carbides, such as the iron carbide cementite in steel, and tungsten carbide,
widely used as an abrasive and for making hard tips for cutting tools.
The system of carbon allotropes spans a range of extremes:
|
Graphite is one of the softest materials known.
|
Synthetic nanocrystalline diamond is the
hardest material known.
|
|
Graphite is a very good lubricant,
displaying superlubricity.
|
Diamond is the ultimate abrasive.
|
|
Graphite is a conductor of electricity.
|
Diamond is an excellent electrical insulator,and
has the highest breakdown electric field of any known material.
|
|
Some forms of graphite are used for thermal insulation (i.e. firebreaks
and heat shields), but some other forms are good thermal
conductors.
|
Diamond is the best known naturally occurring thermal conductor
|
|
Graphite is opaque.
|
Diamond is highly transparent.
|
|
Graphite crystallizes in the hexagonal system.
|
Diamond crystallizes in the cubic system.
|
|
Amorphous carbon is completely isotropic.
|
Carb
|
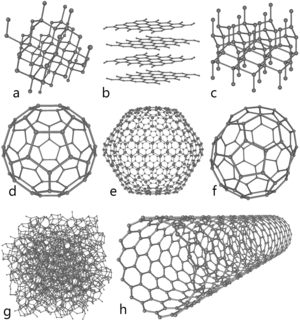
Allotropes
Atomic carbon is a very short-lived species and, therefore, carbon is
stabilized in various multi-atomic structures with different molecular
configurations called allotropes. The three relatively well-known allotropes of
carbon are amorphous carbon, graphite, and diamond. Once considered exotic,
fullerenes are nowadays commonly synthesized and used in research; they include
buckyballs,carbon nanotubes, carbon nanobuds and
nanofibers. Several other exotic allotropes have also been discovered,
such as lonsdaleite (questionable), glassy carbon,carbon nanofoam and linear acetylenic carbon (carbyne).
As of 2009, graphene appears to be the strongest material ever
tested.
The process of separating it from graphite will require some
further technological development before it is economical for industrial
processes.
If successful, graphene could be used in the construction of an
Earth to Space Elevator. It could also be used to safely store hydrogen for use
in a hydrogen based engine in cars.
The amorphous form is an assortment of carbon atoms in a non-crystalline,
irregular, glassy state, not held in a crystalline macrostructure. It is
present as a powder, and is the main constituent of substances such as
charcoal, lampblack (soot) and activated carbon. At normal pressures, carbon
takes the form of graphite, in which each atom is bonded trigonally to three
others in a plane composed of fused hexagonal rings, just like those in
aromatic hydrocarbons.The resulting network is 2-dimensional, and the
resulting flat sheets are stacked and loosely bonded through weak van der Waals
forces. This gives graphite its softness and its cleaving properties (the
sheets slip easily past one another). Because of the delocalization of one of
the outer electrons of each atom to form a π-cloud, graphite conducts
electricity, but only in the plane of each covalently bonded sheet. This
results in a lower bulk electrical conductivity for carbon than for most metals.
The delocalization also accounts for the energetic stability of graphite over
diamond at room temperature.
At very high pressures, carbon forms the more compact allotrope, diamond,
having nearly twice the density of graphite. Here, each atom is bonded
tetrahedrally to four others, forming a 3-dimensional network of puckered
six-membered rings of atoms. Diamond has the same cubic structure as silicon
and germanium, and because of the strength of the carbon-carbon bonds, it is
the hardest naturally occurring substance measured by resistance to scratching.
Contrary to the popular belief that "diamonds are forever", they are
thermodynamically unstable under normal conditions and transform into
graphite.[18] Due to a high activation energy barrier, the transition into
graphite is so slow at normal temperature that it is unnoticeable. Under some
conditions, carbon crystallizes as lonsdaleite, a hexagonal crystal lattice
with all atoms covalently bonded and properties similar to those of
diamond.
Fullerenes are a synthetic crystalline formation with a graphite-like
structure, but in place of hexagons, fullerenes are formed of pentagons (or
even heptagons) of carbon atoms. The missing (or additional) atoms warp the
sheets into spheres, ellipses, or cylinders. The properties of fullerenes
(split into buckyballs, buckytubes, and nanobuds) have not yet been fully
analyzed and represent an intense area of research in nanomaterials. The names
"fullerene" and "buckyball" are given after Richard
Buckminster Fuller, popularizer of geodesic domes, which resemble the structure
of fullerenes. The buckyballs are fairly large molecules formed completely of
carbon bonded trigonally, forming spheroids (the best-known and simplest is the
soccerball-shaped C60 buckminsterfullerene). Carbon nanotubes are
structurally similar to buckyballs, except that each atom is bonded trigonally
in a curved sheet that forms a hollow cylinder. Nanobuds were first
reported in 2007 and are hybrid bucky tube/buckyball materials (buckyballs are
covalently bonded to the outer wall of a nanotube) that combine the properties
of both in a single structure.
Of the other discovered allotropes, carbon nanofoam is a ferromagnetic
allotrope discovered in 1997. It consists of a low-density cluster-assembly of
carbon atoms strung together in a loose three-dimensional web, in which the
atoms are bonded trigonally in six- and seven-membered rings. It is among the
lightest known solids, with a density of about 2 kg/m3.[44] Similarly, glassy carbon
contains a high proportion of closed porosity,[37] but contrary to normal
graphite, the graphitic layers are not stacked like pages in a book, but have a
more random arrangement. Linear acetylenic carbon[39] has the chemical
structure[39] -(C:::C)n-. Carbon in this modification is linear with sp orbital
hybridization, and is a polymer with alternating single and triple bonds. This
carbyne is of considerable interest to nanotechnology as its Young's modulus is
forty times that of the hardest known material – diamond.
In 2015, a team at the North Carolina State University announced the
development of another allotrope they have dubbed Q-carbon, created by a high
energy low duration laser pulse on amorphous carbon dust. Q-carbon is reported
to exhibit ferromagetism, fluorescence, and a hardness superior to
diamonds.
Occurrence
Carbon is the fourth most abundant chemical element in the universe by
mass after hydrogen, helium, and oxygen. Carbon is abundant in the Sun, stars,
comets, and in the atmospheres of most planets. Some meteorites contain
microscopic diamonds that were formed when the solar system was still a
protoplanetary disk.[citation needed] Microscopic diamonds may also be formed
by the intense pressure and high temperature at the sites of meteorite impacts.
tracking polycyclic aromatic hydrocarbons (PAHs) in the universe. More than 20%
of the carbon in the universe may be associated with PAHs, complex compounds of
carbon and hydrogen without oxygen.[49] These compounds figure in the PAH world
hypothesis where they are hypothesized to have a role in abiogenesis and
formation of life. PAHs seem to have been formed "a couple of billion
years" after the Big Bang, are widespread throughout the universe, and are
associated with new stars and exoplanets.
It has been estimated that the solid earth as a whole contains 730 ppm of
carbon, with 2000 ppm in the core and 120 ppm in the combined mantle and
crust.[50] Since the mass of the earth is 5.972×1024 kg, this would imply 4360
million gigatonnes of carbon. This is much more than the amount of carbon in
the oceans or atmosphere (below).
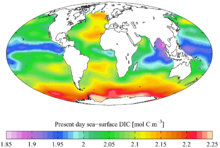
In combination with oxygen in carbon dioxide, carbon is found in the Earth's
atmosphere (approximately 810 gigatonnes of carbon) and dissolved in all water
bodies (approximately 36,000 gigatonnes of carbon). Around 1,900 gigatonnes of
carbon are present in the biosphere. Hydrocarbons (such as coal, petroleum, and
natural gas) contain carbon as well. Coal"reserves" (not
"resources") amount to around 900 gigatonnes with perhaps 18,000 Gt
of resources.[51] Oil reserves are around 150 gigatonnes. Proven sources of
natural gas are about 175 1012 cubic metres (containing about 105 gigatonnes of
carbon), but studies estimate another 900 1012 cubic metres of
"unconventional" deposits such as shale gas, representing about 540
gigatonnes of carbon.[52]
Carbon is also found in methane hydrates in polar regions and under the
seas. Various estimates put this carbon between 500, 2500 Gt,[53] or 3,000
Gt.[54]
In the past, quantities of hydrocarbons were greater. According to one
source, in the period from 1751 to 2008 about 347 gigatonnes of carbon were
released as carbon dioxide to the atmosphere from burning of fossil fuels.[55]
Another source puts the amount added to the atmosphere for the period since
1750 at 879 Gt, and the total going to the atmosphere, sea, and land (such as
peat bogs) at almost 2,000 Gt.[56]
Carbon is a constituent (about 12% by mass) of the very large masses of
carbonate rock (limestone, dolomite, marble and so on). Coal is very rich in
carbon (anthracite contains 92–98%)[57] and is the largest commercial source of
mineral carbon, accounting for 4,000 gigatonnes or 80% of fossil fuel.[58]
As for individual carbon allotropes, graphite is found in large
quantities in the United States (mostly in New York and Texas), Russia, Mexico,
Greenland, and India. Natural diamonds occur in the rock kimberlite, found in
ancient volcanic "necks", or "pipes". Most diamond deposits
are in Africa, notably in South Africa, Namibia, Botswana, the Republic of the
Congo, and Sierra Leone. Diamond deposits have also been found in Arkansas,
Canada, the Russian Arctic, Brazil, and in Northern and Western Australia.
Diamonds are now also being recovered from the ocean floor off the Cape of Good
Hope. Diamonds are found naturally, but about 30% of all industrial diamonds
used in the U.S. are now manufactured.
Carbon-14 is formed in upper layers of the troposphere and the
stratosphere at altitudes of 9–15 km by a reaction that is precipitated by
cosmic rays.[59] Thermal neutrons are produced that collide with the nuclei of
nitrogen-14, forming carbon-14 and a proton. As such, 1.2 × 1010% of atmospheric
carbon dioxide contains carbon-14.[24]
Carbon-rich asteroids are relatively preponderant in the outer parts of
the asteroid belt in our solar system. These asteroids have not yet been
directly sampled by scientists. The asteroids can be used in hypothetical
space-based carbon mining, which may be possible in the future, but is
currently technologically impossible.[60]
Isotopes
Main article: Isotopes of carbon
Isotopes of carbon are atomic nuclei that contain six protons plus a
number of neutrons (varying from 2 to 16). Carbon has two stable, naturally
occurring isotopes.[15] The isotope carbon-12 (12C) forms 98.93% of the carbon
on Earth, while carbon-13 (13C) forms the remaining 1.07%.[15] The
concentration of 12C is further increased in biological materials because
biochemical reactions discriminate against 13C.[61] In 1961, the International
Union of Pure and Applied Chemistry (IUPAC) adopted the isotope carbon-12 as
the basis for atomic weights.[62] Identification of carbon in nuclear magnetic
resonance (NMR) experiments is done with the isotope 13C.
Carbon-14 (14C) is a naturally occurring radioisotope, created in the
upper atmosphere (lower stratosphere and upper troposphere) by interaction of
nitrogen with cosmic rays.[63] It is found in trace amounts on Earth of up to 1
part per trillion (0.0000000001%), mostly confined to the atmosphere and
superficial deposits, particularly of peat and other organic materials.[64]
This isotope decays by 0.158 MeV β− emission. Because of its relatively short
half-life of 5730 years, 14C is virtually absent in ancient rocks. The amount
of 14C in the atmosphere and in living organisms is almost constant, but
decreases predictably in their bodies after death. This principle is used in
radiocarbon dating, invented in 1949, which has been used extensively to
determine the age of carbonaceous materials with ages up to about 40,000
years.[65][66]
There are 15 known isotopes of carbon and the shortest-lived of these is
8C which decays through proton emission and alpha decay and has a half-life of
1.98739x10−21 s.[67] The exotic 19C exhibits a nuclear halo, which means its
radius is appreciably larger than would be expected if the nucleus were a
sphere of constant density.[68]
Formation in stars
Formation of the carbon atomic nucleus requires a nearly simultaneous
triple collision of alpha particles (helium nuclei) within the core of a giant
or supergiant star which is known as the triple-alpha process, as the products
of further nuclear fusion reactions of helium with hydrogen or another helium
nucleus produce lithium-5 and beryllium-8 respectively, both of which are
highly unstable and decay almost instantly back into smaller nuclei. This
happens in conditions of temperatures over 100 megakelvin and helium
concentration that the rapid expansion and cooling of the early universe
prohibited, and therefore no significant carbon was created during the Big
Bang.
According to current physical cosmology theory, carbon is formed in the
interiors of stars in the horizontal branch by the collision and transformation
of three helium nuclei.[70] When those stars die as supernova, the carbon is
scattered into space as dust. This dust becomes component material for the
formation of second or third-generation star systems with accreted
planets. The Solar System is one such star system with an abundance of
carbon, enabling the existence of life as we know it.
The CNO cycle is an additional fusion mechanisms that powers stars,
wherein carbon operates as a catalyst.
Rotational transitions of various isotopic forms of carbon monoxide (for
example, 12CO, 13CO, and 18CO) are detectable in the submillimeter wavelength
range, and are used in the study of newly forming stars in molecular
clouds.
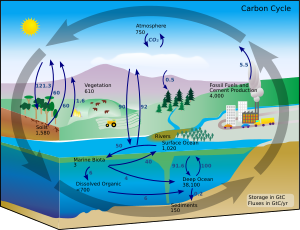
Carbon cycle
Under terrestrial conditions, conversion of one element to another is
very rare. Therefore, the amount of carbon on Earth is effectively constant.
Thus, processes that use carbon must obtain it from somewhere and dispose of it
somewhere else. The paths of carbon in the environment form the carbon cycle.
For example, photosynthetic plants draw carbon dioxide from the atmosphere (or
seawater) and build it into biomass, as in the Calvin cycle, a process of
carbon fixation. Some of this biomass is eaten by animals, while some carbon is
exhaled by animals as carbon dioxide. The carbon cycle is considerably more
complicated than this short loop; for example, some carbon dioxide is dissolved
in the oceans; if bacteria do not consume it, dead plant or animal matter may
become petroleum or coal, which releases carbon when burned.
Compounds
Organic compounds
Carbon can form very long chains of interconnecting carbon–carbon bonds,
a property that is called catenation. Carbon-carbon bonds are strong and
stable. Through catenation, carbon forms a countless number of compounds. A
tally of unique compounds shows that more contain carbon that those that do
not.[citation needed] A similar claim can be made for hydrogen because most
organic compounds also contain hydrogen.[citation needed]
The simplest form of an organic molecule is the hydrocarbon—a large
family of organic molecules that are composed of hydrogen atoms bonded to a
chain of carbon atoms. Chain length, side chains and functional groups all
affect the properties of organic molecules.
Carbon occurs in all known organic life and is the basis of organic
chemistry. When united with hydrogen, it forms various hydrocarbons that are
important to industry as refrigerants, lubricants, solvents, as chemical
feedstock for the manufacture of plastics and petrochemicals, and as fossil
fuels.
When combined with oxygen and hydrogen, carbon can form many groups of
important biological compounds including sugars, lignans, chitins, alcohols, fats,
and aromatic esters, carotenoids and terpenes. With nitrogen it forms
alkaloids, and with the addition of sulfur also it forms antibiotics, amino
acids, and rubber products. With the addition of phosphorus to these other
elements, it forms DNA and RNA, the chemical-code carriers of life, and
adenosine triphosphate (ATP), the most important energy-transfer molecule in
all living cells.
Inorganic compounds
Commonly carbon-containing compounds which are associated with minerals
or which do not contain hydrogen or fluorine, are treated separately from
classical organic compounds; the definition is not rigid (see reference
articles above). Among these are the simple oxides of carbon. The most
prominent oxide is carbon dioxide (CO2). This was once the principal
constituent of the paleoatmosphere, but is a minor component of the Earth's
atmosphere today. Dissolved in water, it forms carbonic acid (H
2CO
3), but as most compounds with multiple single-bonded oxygens on a single
carbon it is unstable.[76] Through this intermediate, though,
resonance-stabilized carbonate ions are produced. Some important minerals are
carbonates, notably calcite. Carbon disulfide (CS
2) is similar.
The other common oxide is carbon monoxide (CO). It is formed by
incomplete combustion, and is a colorless, odorless gas. The molecules each
contain a triple bond and are fairly polar, resulting in a tendency to bind
permanently to hemoglobin molecules, displacing oxygen, which has a lower
binding affinity. Cyanide (CN−), has a similar structure, but behaves
much like a halide ion (pseudohalogen). For example, it can form the nitride
cyanogen molecule ((CN)2), similar to diatomic halides. Other uncommon oxides
are carbon suboxide (C
3O
2),[79] the unstable dicarbon monoxide (C2O),[80][81] carbon trioxide
(CO3),[82][83]cyclopentanepentone (C5O5),[84] cyclohexanehexone (C6O6),[84] and
mellitic anhydride (C12O9).
With reactive metals, such as tungsten, carbon forms either carbides
(C4−) or acetylides (C2−
2) to form alloys with high melting points. These anions are also
associated with methane and acetylene, both very weak acids. With an
electronegativity of 2.5,[85] carbon prefers to form covalent bonds. A few
carbides are covalent lattices, like carborundum (SiC), which resembles
diamond. Nevertheless, even the most polar and salt-like of carbides are not
completely ionic compounds.
Organometallic compounds
Organometallic compounds by definition contain at least one carbon-metal
bond. A wide range of such compounds exist; major classes include simple
alkyl-metal compounds (for example, tetraethyllead), η2-alkene compounds (for
example, Zeise's salt), and η3-allyl compounds (for example, allylpalladium
chloride dimer); metallocenes containing cyclopentadienyl ligands (for example,
ferrocene); and transition metal carbene complexes. Many metal carbonyls exist
(for example, tetracarbonylnickel); some workers consider the carbon monoxide
ligand to be purely inorganic, and not organometallic.
While carbon is understood to exclusively form four bonds, an interesting
compound containing an octahedral hexacoordinated carbon atom has been
reported. The cation of the compound is [(Ph3PAu)6C]2+. This phenomenon has
been attributed to the aurophilicity of the gold ligands.
In 2016, it was confirmed that hexamethylbenzene contains a carbon atom
with six bonds, rather than the usual four.
















Nice blog!!
ReplyDeleteI like your content and you can also read our latest blog here C45 Material
ReplyDeleteI like your work!
ReplyDeleteWe appreciate your work and you can also read our interesting blog here EN24 ALLOY STEEL
ReplyDelete